
Basic information
Magnesium (Mg) is an alkaline earth element with smaller ionic radius (0.80 A) than calcium, but similar to that of iron. The highest concentrations therefore are found in minerals (amphiboles, pyroxenes, olivine and biotite ) in mafic and ultramafic igneous rocks. In sedimentary environments magnesium forms the mineral dolomite (CaMg(CO3) 2) and importantly in relation to groundwaters, may substitute in calcites from trace amounts up to 18 mole %.
Measurement techniques
Mg2+ can be measured using either ion chromatography (IC) or inductively coupled plasma optical emission spectroscopy (ICP-OES)
Applications
The ratio Mg/Ca is one of the most important in hydrogeochemistry and may be used as a tracer of water origin and qualitatively as residence time indicator. The value as tracer stems from its distinctly high concentration in sea water (m Mg/Ca 5.43) and in the various mineral-water interactions. These are best illustrated with the help of the trilinear diagram, which is based on the Chalk aquifer in UK.
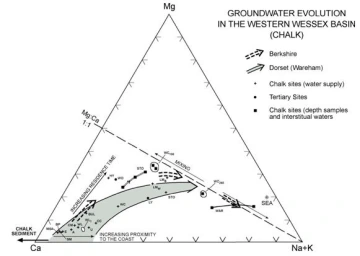
Trilinear diagram summarizing reactions in carbonate aquifers (using the example of the UK Chalk (based on Edmunds et al 2008)
This diagram shows the Mg-rich composition of sea water. It also shows the Mg/Ca 1:1 line which should be the composition indicating equilibrium with dolomite. Groundwater initially shows the Ca-rich composition derived from congruent (rapid) dissolution of low-Mg calcite. With continuing evolution water-rock interaction progressively releases Mg impurity from the chalk sediment incongruent solution) and some mixing occurs with saline water. The trend continues until a point where it becomes parallel with the one to one line (equilibrium with dolomite (and calcite). This trend is an indicator of residence time in most carbonate aquifers.
In Mg-rich lithologies (such as basic igneous rocks) it is likely that Mg/Ca ratios in excess of 1 will be found. During modern sea water intrusion the Mg/Ca ratio may be used to estimate mixing, although with increasing residence time considerable water-rock interaction (clay mineral formation) will lower the initial ratio.
References
- Edmunds, W.M., Cook, J.M., Darling, W.G., Kinniburgh, D.G., Miles, D.L., Bath, A.H., Morgan-Jones, M. and Andrews, J.N., 1987. Baseline geochemical conditions in the Chalk aquifer, Berkshire, UK: a basis for groundwater quality management, Applied Geochemistry 2, 251–274.
