Hydrogen has three isotopes, two stable (1H and 2H), and one radioactive (3H). The stable isotopes are considered together with oxygen. The radioactive isotope tritium (3H) is considered here. It can be used for dating very young groundwaters (less than 50 years).
Cost of Analysis
Direct Counting: $175; turn around time is 1 to 2 months.
Mass Spectrometry: $300
Origin
Cosmogenic
Natural tritium is created in the upper atmosphere from the cosmic bombardment of nitrogen with neutrons. Tritium then combines with oxygen to produce tritiated water (H3HO) and enters the hydrologic cycle.
Tritium decays to a rare, stable isotope of helium (3He) by beta emission.
Lithogenic
Lithogenic tritium is produced by the showering of lithium present in rocks by neutrons produced during the spontaneous fission of uranium and thorium.
This process is limited by the amount of lithium in rocks. In most cases, lithogenic production is negligible compared to other sources. The lithogenic tritium enters the groundwater directly.
Anthropogenic
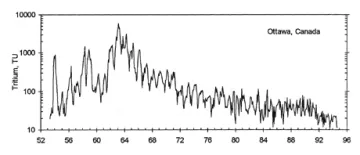
The figure above illustrates the monthly levels of tritium in precipitation at Ottawa, Canada, the longest existing record. (Reprinted from Clark and Fritz 1997, p. 178; monitoring record established by R.M. Brown, AECL, Canada).
Natural production of tritium in the atmosphere is very low. Due to the advent of thermonuclear technology, this production in the atmosphere has been supplemented by anthropogenic production. Beginning in the 1950's, large amounts were produced from the atmospheric testing of thermonuclear bombs,. Concentrations of tritium decreased after 1963 because the United States, the USSR, and the United Kingdom signed the Soviet-American treaty banning above-ground testing. Because anthropogenic tritium is also currently produced by releases from nuclear power plants, present day concentrations of tritium in the atmosphere have not returned to natural concentrations, but levels have gradually decreased since the 1960's as the tritium continues to decay.
All atmospheric tritium, whether cosmogenic or anthropogenic, is rapidly incorporated into water molecules and falls in meteoric precipitation to enter the hydrologic cycle.
Measurement Techniques
Tritium concentrations are represented in tritium units (TU). One tritium unit is equal to one molecule of 3H per 1018 molecules of 1H and has an activity of 0.118 Bq/kg (3.19 pCi/kg).
Decay-counting
Tritium is typically measured by a liquid scintillation counter. The sample is mixed with a scintillation mixture of a solvent, emulsifier, and solute. Tritium emits beta decay electrons, which excites the solvent. The solvent transfers its energy to the solute, which emits light photons. The light pulses are detected and counted.
Mass spectrometry
Tritium and its daughter 3He can be measured using mass spectrometry, but other dissolved gases (H2O, CO2, O2, N2, etc.) must be removed first. Lower detection limits can be achieved by mass spectrometric measurements of 3He produced by the "ingrowth method," where the tritium sample is stripped of all gases and then stored for a sufficient time for enough daughter product 3He to be produced and measured. The mass spectrometer method has the advantage over the liquid scintillation method because both 3He ingrowth (3H) and the 3He of a sample can be measured. This provides quantitative age determinations discussed in the section below.
Hydrological Applications
The large pulse of tritium that entered the hydrologic cycle in the 1960's can be used to establish the age of recent groundwater recharge. High levels of tritium (>~30 TU) indicate water that was recharged during the late 1950's or early 1960's; moderate concentrations indicate modern recharge; levels close to detection (~1 TU) are likely submodern or paleogroundwaters that have mixed with shallow modern groundwaters. (Clark and Fritz 1997). General guidelines are:
| <0.8 TU | submodern (prior to 1950s) |
| .8 - 4 TU | mix of submodern and modern |
| 5 - 15 TU | modern (<5 to 10 years) |
| 15 - 30 TU | some bomb tritium |
| >30 TU | recharge in the 1960's to 1970's |
| >50 TU | recharge in the 1960' |
Bomb-produced tritium can be used as a tracer in studying young groundwaters to help determine flow rates and directions, mean residence times, and hydraulic parameters such as conductivity, and can also be helpful in observing preferential flow paths and in investigating the mixing of waters. Its use, however, is somewhat limited by a number of factors, including uneven global distribution and local variations due to continued nuclear releases. Some approaches that may be used to counter the problems using 3H in dating groundwater include using time series analysis to monitor the bomb spike for 3H in an aquifer to provide an indication of mean residence time.
Groundwater can also be aged quantitatively using tritium and its daughter,3He. Age is determined by:
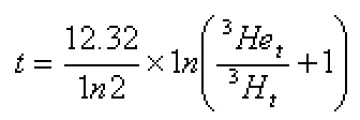
where t is the time since isolation from contact with the atmosphere after decay (estimated age), and 3Het/3Ht is the concentration ratio of the two isotopes expressed in TUs.
Aging by the 3H-3He method also presents problems, since the total 3He in groundwater comes from a variety of sources: the atmosphere, 3H decay, subsurface nuclear reactions, and the earth's mantle. The measured concentration of 3He must be corrected for these other sources. 3He is also not a routinely sampled or measured isotope. Other concerns are the fractionation of 3He if a gas phase is present and the fact that the solubility of He is temperature dependent.
References and Further Reading
- Faure, G., Principles of Isotope Geology, 2nd ed., John Wiley and Sons, New York, 1986.
- Clark, I., and P. Fritz, Environmental Isotopes in Hydrogeology, Lewis Publishers, Boca Raton, 1997.
- Gonfiantini, R, Environmental isotopes in lake studies, in Handbook of Environmental Isotope Geochemistry, vol. 2, edited by P. Fritz and J.-Ch. Fontes, pp. 113-168, Elsevier, Amsterdam, 1986.
Internet Resources
- International Atomic Energy Agency, Isotope Hydrology Information System (ISOHIS)
- International Atomic Energy Agency, The Development of GNIP (Global Network of Isotopes in Precipitation)
- USGS Periodic Table - Hydrogen
